Iwai North America Inc.
541 Taylor Way Suite# 4
San Carlos, CA 94070
Phone : (650) 486-1541
Fax : (650) 394-8638
Open weekdays 9 AM-6 PM (PST)
.........................................................................................................................................................................................................................................................
Have questions about our products?
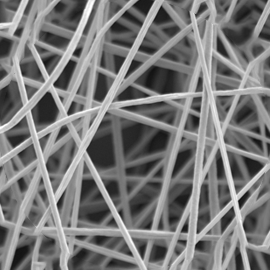
Cellevate 3D Nanomatrix is the next generation cell culture system. It is highly porous, and its patented manufacturing process enables a consistent network of nanofibers.
What is Cellevate technology?
Nanofibers, usually defined as fibers with diameter on the nanoscale (1-1000nm), have during the last decade received great attention both in academia and industry. The large surface to volume ratio generates several interesting properties which are desirable for a variety of applications. Although this type of material and its applications are still in their cradle, the industry and academia have already begun to explore its possibilities and significant advancements have been made within well-established industries.
Cellevate NanoMatrix is a nanofiber scaffold made from fully synthetic polycarprolactone (PCL) for 3D cell culture. Produced through a patented technique in which we revised the standard electrospinning method used by others, and improved scaffold parameter control (subsequently the overall fiber quality) and increased batch-to-batch consistency.
 | ||||||
Features of Celevate NanoMatrix
- Biomimics the extracellular matrix (ECM). Porous, elastic, etc.
- Mechanical Properties are customizable (nanofibers, porosity, elasticity, etc.)
- Electrospun scaffold allows for batch-to-batch consistency.
- Flexibility in functionalization. Example: Coat laminin to the nanofiber
- Co-Culturable
- Viability of cells for long term cell culture
- Alternative to Animal Testing
Proof of Concept
Protocols & General instruction of use
How to & Protocols
Ready to Use Models
Breast Cancer
In a collaboration with Lund University, we have developed a scaffold for co-culture of breast cancer research.
We have successfully demonstrated that different cell types, malignant as well as normal thrive in the 3D fibre unit. We are presently exploring this model for co-culturing of cancer cells and different types of stromal cells. We anticipate this model can be used to create a 3D tumour ex vivo platform that can be used for screening of therapeutic compounds, which will reduce the number of animals in cancer research.
Cells Tested:
- L929(mouse) and human fibroblasts
- MCF-7 and JIMT-1 (human breast cancer cell lines)
- MCF-10A (normal-like human breast cell line)
References:
- Permlid et al, 2019
- Permlid et al, 2021
Pancreatic Cancer
In a collaboration with Swedish CRO, we have developed a scaffold for stable, long-term co-culture of pancreatic cancer and stromal cells. Their model for pancreatic cancer co-cultures, a traditional submerged 2D system had performance issues. Limited space meant contact inhibition was problematic and the system became over confluent around 4 DIV, which was not enough time to develop a disease relevant culture. The model is currently being used to further investigate drug sensitivity and cytotoxicity.
Cells Tested:
- AsPc1
- CAFs
References:
Neurodegenerative Diseases: iPSC culture for psychiatric disease
In a collaboration with European Pharmaceutical company, we have developed a scaffold for culture of iPSCs for studying psychiatric disease. Neurons and astrocytes matured faster in 3D culture compared to conventional 2D culture and the co-cultures could be maintained for several weeks without affecting cell viability. First staining indicate that neurons develop a very dense neuronal network and surrounding astrocytes are in close interaction with them.
Cells Tested:
- iPSC, iNeuron
References:
Neurodegenerative Diseases: HNPC culture for basic research
In a collaboration with Lund University, we have developed a scaffold for suitable for both human neuronal progenitor cells (HNPCs) and retinal cells.
Successfully, we here demonstrate a functional and true 3D neuronal network after seeding of human neural progenitors into uncompressed highly porous electrospun PCL fiber scaffold. Notably, glial cells were found integrated in 3D in an in vivo mimicking way — both in the scaffolds as well as with neighboring neurons. Further exploration of such uncompressed electrospun scaffolds is highly motivated, with different neural tool cells for both basic neurobiology studies and in neural engineering approaches where they can be investigated for support and axonal guidance upon implantation into the nervous system.
Cells Tested:
- neuronal progenitor cells (HNPCs)
- retinal
References:
- Zalis, 2016
- Jakobson, 2017
- Zalis, 2018
Customization
Cellevate uses networks of nanofibers to mimic different types of tissue. Our strength lies in our unique technology, combined with years of know-how and expertise, that allows us to fine tune the parameters of the nanomaterials we create and offer our customers tailor made solutions optimized for your specific applications.
References
Atena Malakpour Permlid, Plaurent Roci, Elina Fredlund, Felicia Fält, Emil Önell, Fredrik Johansson, Stina Oredsson, Unique animal friendly 3D culturing of human cancer and normal cells, Toxicology in Vitro, Volume 60, 2019, Pages 51-60, ISSN 0887-2333, https://doi.org/10.1016/j.tiv.2019.04.022.
Dahlin L.B., Stenberg L., Johansson U.E., Johansson F. (2019) Traumatic Peripheral Nerve Injuries: Experimental Models for Repair and Reconstruction. In: Risling M., Davidsson J. (eds) Animal Models of Neurotrauma. Neuromethods, vol 149. Humana, New York, NY. https://doi.org/10.1007/978-1-4939-9711-4_9
Frost, H.K., Andersson, T., Johansson, S. et al. Electrospun nerve guide conduits have the potential to bridge peripheral nerve injuries in vivo. Sci Rep 8, 16716 (2018). https://doi.org/10.1038/s41598-018-34699-8
Zalis MC, Nylander M, Enjin A, Stensmyr MC, Johansson P, O'Carroll D and Englund Johansson U (2019). Assessing electrical activity of human neuronal cell culture networks grown on 3D fibrous scaffolds using different techniques: A comparative study. Conference Abstract: MEA Meeting 2018 | 11th International Meeting on Substrate Integrated Microelectrode Arrays. doi: 10.3389/conf.fncel.2018.38.00048
Albin Jakobsson, Maximilian Ottosson, Marina Castro Zalis, David O'Carroll, Ulrica Englund Johansson, Fredrik Johansson, Three-dimensional functional human neuronal networks in uncompressed low-density electrospun fiber scaffolds, Nanomedicine: Nanotechnology, Biology and Medicine, Volume 13, Issue 4, 2017, Pages 1563-1573, ISSN 1549-9634, https://doi.org/10.1016/j.nano.2016.12.023.
Englund-Johansson, U. , Netanyah, E. and Johansson, F. (2017) Tailor-Made Electrospun Culture Scaffolds Control Human Neural Progenitor Cell Behavior—Studies on Cellular Migration and Phenotypic Differentiation. Journal of Biomaterials and Nanobiotechnology, 8, 1-21. doi: 10.4236/jbnb.2017.81001.
Ottosson M, Jakobsson A, Johansson F (2017) Accelerated Wound Closure - Differently Organized Nanofibers Affect Cell Migration and Hence the Closure of Artificial Wounds in a Cell Based In Vitro Model. PLoS ONE 12(1): e0169419. https://doi.org/10.1371/journal.pone.0169419
Marina Castro Zalis, Sebastian Johansson, Fredrik Johansson, Ulrica Englund Johansson, Exploration of physical and chemical cues on retinal cell fate, Molecular and Cellular Neuroscience, Volume 75, 2016, Pages 122-132, ISSN 1044-7431, https://doi.org/10.1016/j.mcn.2016.07.006.