SHIKARI® Q-CERT
Enzyme immunoassay for the quantitative determination of Certolizumab(CIMZIA®) in serum and plasma. The Matriks Biotek Shikari Q-CERT ELISA has been especially developed for the quantitative analysis of free Certolizumab in serum and plasma samples.
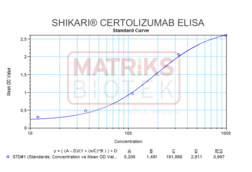
Calibration Curve
SHIKARI® Q-CERT
Enzyme immunoassay for the quantitative determination of Certolizumab(CIMZIA®) in human serum and plasma
CER-FD-CIM: $945.00 Lead time: 1-2 weeks
 | ||||
.....................................................
.....................................................
Iwai North America Inc.
541 Taylor Way Suite# 4
San Carlos, CA 94070
Phone : (650) 486-1541
Fax : (650) 394-8638
Open weekdays 9 AM-6 PM (PST)
.........................................................................................................................................................................................................................................................
Required Volume
Total Time
Sample
Sample Number
Detection Limit
Spike Recovery
Shelf Life
10ul
70 min
Aqueous humour
96
37 ng/mL
85-115%
1 year