SHIKARI® Q-DEN
The Matriks Biotek Denosumab ELISA has been specially developed for the quantitative analysis of Denosumab in serum and plasma samples between the Cmin and Cmax range of concentrations (indicated in the pharmacokinetics section of the protocol linked below) .
SHIKARI® S-ATD
Required Volume
Total Time
Sample
Sample Number
Detection Limit
Spike Recovery
Shelf Life
10ul
70 min
Serum, plasma
96
3ng/mL
85-115%
1 year
The Matriks Biotek anti-Denosumab (Prolia®) Enzyme-Linked-ImmunoSorbentAssay (ELISA) Kit is intended for the qualitative determination of antibodies to Denosumab (Prolia®) in serum and plasma.
It is for professional use only.
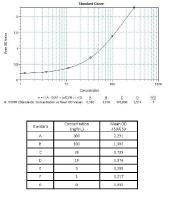
Calibration Curve
SHIKARI® Q-DEN & S-ATD
Denosumab (Prolia®)
Enzyme immunoassay for the quantitative determination of Denosumab (Prolia®) in serum and plasma
Enzyme immunoassay for the qualitative determination (screening) of antibodies to Denosumab (Prolia®) in serum and plasma
Required Volume
Total Time
Sample
Sample Number
Detection Limit
Spike Recovery
Shelf Life
10ul
140 min
Serum, plasma
96
+/-
-
1 year
DEN-FD-PRO: $1000.00 Lead time: 1-2 weeks
DEN-QLS-PRO: $1000.00 Lead time: 1-2 weeks
 | ||||
.....................................................
.....................................................
Drug Measurement
Anti-drug Antibody Measurement
Quantitative
Qualitative
Denosumab (Prolia®)
Osteoporosis Drugs
Iwai North America Inc.
541 Taylor Way Suite# 4
San Carlos, CA 94070
Phone : (650) 486-1541
Fax : (650) 394-8638
Open weekdays 9 AM-6 PM (PST)
.........................................................................................................................................................................................................................................................