SHIKARI® Q-PEM
Matriks Biotek® pembrolizumab ELISA has been specially developed for the quantitative analysis of Pembrolizumab in serum and plasma samples between the Cmin and Cmax range of concentrations (indicated in the pharmacokinetics section of protocol linked below).
SHIKARI® S-ATP
Required Volume
Total Time
Sample
Sample Number
Detection Limit
Spike Recovery
Shelf Life
10ul
140 min
Serum, plasma
96
30ng/mL
85-115%
1 year
The Matriks Biotek anti-Pembrolizumab (Keytruda®) Enzyme-Linked ImmunoSorbentAssay (ELISA) Kit is intended for the qualitative determination of antibodies to Pembrolizumab (Keytruda®) in serum and plasma. It is for professional use only.
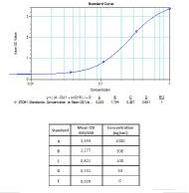
Calibration Curve
SHIKARI® Q-PEM & S-ATP
Pembrolizumab (Keytruda®)
Enzyme immunoassay for the quantitative determination of Pembrolizumab (Keytruda®) in serum and plasma
Enzyme immunoassay for the qualitative determination (screening) of antibodies to Pembrolizumab (Keytruda®) in serum and plasma
Required Volume
Total Time
Sample
Sample Number
Detection Limit
Spike Recovery
Shelf Life
10ul
140 min
Serum, plasma
96
+/-
-
1 year
PEM-FD-KEY: $1000.00 Lead time: 1-2 weeks
PEM-QLS-KEY: $945.00 Lead time: 1-2 weeks
 | ||||
.....................................................
.....................................................
Drug Measurement
Anti-drug Antibody Measurement
Quantitative
Qualitative
Pembrolizumab (Keytruda®)
PD-1, PD-L1 and CTLA-4
Iwai North America Inc.
541 Taylor Way Suite# 4
San Carlos, CA 94070
Phone : (650) 486-1541
Fax : (650) 394-8638
Open weekdays 9 AM-6 PM (PST)
.........................................................................................................................................................................................................................................................