SHIKARI® Q-REMS
The Committee for Medicinal Products for Human Use (CHMP) has stated that evidence shows Remsima® (the world's first biosimilar mAb) to be of comparable quality, safety and efficacy as Remicade®. The SHIKARI® Infliximab-Remsima® ELISA has been specially developed for the quantitative analysis of free Infliximab in serum and plasma samples.
SHIKARI® S-AIR
Required Volume
Total Time
Sample
Sample Number
Detection Limit
Spike Recovery
Shelf Life
10ul
70 min
Serum, plasma
96
100ng/mL
85-115%
1 year
The use of Infliximab (Remsima) has been linked to the development of antibodies against the drug at various levels, possibly leading to severe complications and even resistance to the treatment. The SHIKARI® S-AIR ELISA Kit provides a tool for easily monitoring antibodies to Infliximab Remsima and exploring ways of limiting problematic immunoresponses.
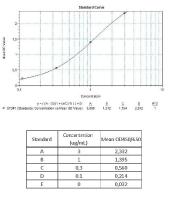
Calibration Curve
SHIKARI® Q-REMS & S-AIR
Infliximab Biosimilar (Remsima®)
Enzyme immunoassay for the quantitative determination of free Infliximab (Remsima®) in serum and plasma
Enzyme immunoassay for the determination of antibodies to Infliximab Remsima® in serum and plasma
Required Volume
Total Time
Sample
Sample Number
Detection Limit
Spike Recovery
Shelf Life
20ul
140 min
Serum, plasma
96
30ng/ml
85-115%
1 year
INF-FD-REMS: $910.00 Lead time: 1-2 weeks
INF-QLS-REMS (w/o Confirmation): $910.00
INF-QNS-REMS (w/ Confirmation) : $945.00
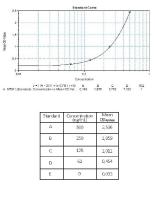
Calibration Curve
INF-QLS-REMS
INF-QNS-REMS
Peer Reviewed Journals:
5 Gibellini L, De Biasi S, Bianchini E, et al. Anti-TNF-α Drugs Differently Affect the TNFα-sTNFR System and Monocyte Subsets in Patients with Psoriasis. Richard Y, ed. PLoS ONE. 11(12), 2016.**
8 Choi SY, Kang B, Lee JH, Choe YH. Clinical Use of Measuring Trough Levels and Antibodies against Infliximab in Patients with Pediatric Inflammatory Bowel Disease. Gut Liver. Sep 9 2016.**
12 Won Jae Song, Ben Kang, So Yoon Choi, and Yon Ho Choe. Adalimumab Treatment in Pediatric-Onset Crohn’s Disease Patients after Infliximab Failure: A Single Center Study. Pediatr Gastroenterol Hepatol Nutr. Jun; 19(2): 116–122, 2016.**
13 Hayashi S, et al., Early Prognostic Factors Associated with the Efficacy of Infliximab Treatment for Patients with Rheumatoid Arthritis with Inadequate Response to Methotrexate. Rheumatol Ther (2016) 3:155–166 **
15 Al-Karkhi M.A., et al., Correlation between Anti-infliximab and Anti-CCP Antibodies Development in Patients with Rheumatoid Arthritis Treated with Infliximab in Baghdad Teaching Hospital. IOSR Journal of Dental and Medical Sciences, Volume 14, Issue 11 Ver. IV (Nov. 2015), PP 95-100.**
23 Al-Karkhi M.A., et al., Development of Antibodies against Infliximab in Iraqi Patients with Rheumatoid Arthritis. J Fac Med Baghdad, 57: (241-243), 2015.**
26 Pallagi-Kunstár É. et al., Utility of serum TNF-a, infliximab trough level, and antibody titers in inflammatory bowel disease. World J Gastroenterol. 20(17): (5031-5035), 2014. **
27 Khanna R., et al., Therapeutic Drug Monitoring of TNF Antagonists in Inflammatory Bowel Disease. Gastroenterology & Hepatology, August (478-489),2014. **
33 Gutierrez A, et al, Genetic susceptibility to increased bacterial translocation influences the response to biological therapy in patients with Crohn’s disease, Gut 0:1–9, 2013. **
34 Grosen A., et al, Infliximab concentrations in the milk of nursing mothers with inflammatory bowel disease, J Crohns Colitis 2013. **
38 Bortlik M, et al, Infliximab trough levelsmay predict sustained response to infliximab in patients with Crohn's disease, Journal of Crohn's and Colitis 2012. **
39 Malickova K, et al, Phosphatidylserine-dependent anti-prothrombin antibodies (aPS/PT) in infliximab-treated patients with inflammatory bowel diseases, Autoimmun Highlights, 2012. **
40 Takahashi H, et al, Plasma trough levels of adalimumab and infliximab in terms of clinical efficacy during the treatment of psoriasis, Journal of Dermatology 2012; 39: 1- 4. **
41 Seok Lee Y, et al, “Efficacy of Early Infliximab Treatment for Pediatric Crohn’s Disease: A Three-year Follow-up”, Pediatric Gastroenterology, Hepatology & Nutrition 2012; 15(4):243-249 **
** Open Access
 | ||||
.....................................................
Lead time: 1-2 weeks
.....................................................
Drug Measurement
Anti-drug Antibody Measurement
Quantitative
Quantitative
Qualitative
Semi-Quantitative
Infliximab Biosimilar (Remsima®)
Anti TNF-alpha Blockers
Iwai North America Inc.
541 Taylor Way Suite# 4
San Carlos, CA 94070
Phone : (650) 486-1541
Fax : (650) 394-8638
Open weekdays 9 AM-6 PM (PST)
.........................................................................................................................................................................................................................................................
SHIKARI® S-AIR(v3) T
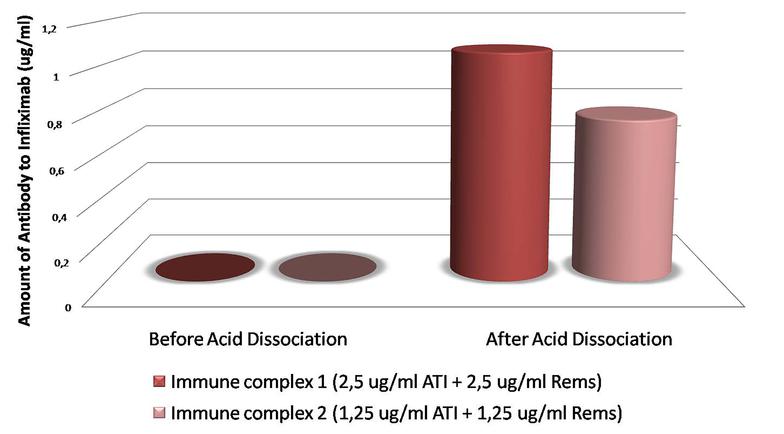
Demonstration of anti-infliximab antibodies during treatment with infliximab (Remsima®) has been a major concern for the new technology of biosimilar mAbs, and monitoring for the presence and/or quantitation of specific antibodies during clinical trials is an important issue for follow up evaluations of treatment regimens. With the Matriks Biotek S-AIR-T ELISA Kit infiliximab-specific antibodies that are bound to infiliximab in serum and can not be detected by free antibody detection kits can be determined in patients receiving Remsima®.
Enzyme immunoassay for the semi-quantitative determination (screening) of total antibodies to infliximab biosimilar (Remsima®) in serum and plasma
Required Volume
Total Time
Sample
Sample Number
Detection Limit
Spike Recovery
Shelf Life
10ul
95 min
Serum, plasma
96
156ng/ml
-
1 year
INF-QNT-REMS (Semi-quantitative): $943.00
Lead time: 1-2 weeks