SHIKARI® Q-BEVA
The levels of free Bevacizumab in serum and the monitoring thereof during maintenance therapy could indicate possible outcomes and the potential for other side effects. In this context, the Q-BEVA Kit is a useful tool for identifying biomarkers for (non-) response and risk factors for adverse drug reactions, as well as establishing an effective minimum concentration.
SHIKARI® S-ATB
Required Volume
Total Time
Sample
Sample Number
Detection Limit
Spike Recovery
Shelf Life
5ul
70 min
Serum, plasma
96
30ng/mL
85-115%
1 year
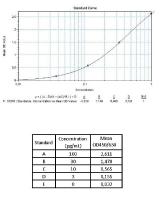
Calibration Curve
SHIKARI® Q-BEVA & S-ATB
Bevacizumab (Avastin®)
Enzyme immunoassay for the quantitative determination of Bevacizumab (Avastin®) in serum and plasma
Enzyme immunoassay for the determination of antibodies to Bevacizumab (Avastin®) in serum and plasma.
Required Volume
Total Time
Sample
Sample Number
Detection Limit
Spike Recovery
Shelf Life
10ul
140 min
Serum, plasma
96
30ng/ml
85-115%
1 year
BEV-FD-AVA: $910.00 Lead time: 2-3 weeks
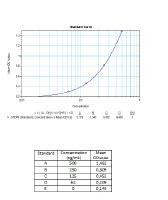
Calibration Curve
BEV-QNS-AVA
BEV-QLS-AVA
Peer Reviewed Journals:
Chen L., et al., Efficient Production of a Bioactive Bevacizumab Monoclonal Antibody Using the 2A Self-cleavage Peptide in Transgenic Rice Callus. Frontiers in Plant Science August 2016 | Volume 7 | Article 1156.**
Bergen T.V., et al., Complementary effects of bevacizumab and MMC in the improvement of surgical outcome after glaucoma filtration surgery. Acta Ophthalmologica 2015, 667-678.**
PhD Theses:
"Evaluation of Transscleral Permeability Effects of Nanotechnology Based Drug Delivery System in Ocular Drug Applications"
By Dr. NAGİHAN UĞURLU, Hacettepe University
(In Turkish with English abstract.)
As with all therapeutic proteins, there is a potential for immunogenicity towards Bevacizumab (Avastin®). However, according to the manufacturer's product insert, the incidence of antibody development has not been adequately determined because the assay sensitivity was inadequate to reliably detect lower titers. Immunogenicity data are highly dependent on the sensitivity and specificity of the assay, and the observed incidence of antibody positivity in an assay may be influenced by several factors, including sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Avastin with the incidence of antibodies to other products may be misleading. However, the SHIKARI® S-ATB Kit provides a tool for easily monitoring anti-Bevacizumab antibodies and exploring ways of understanding problematic immunoresponses to this drug.
* AVASTIN® is a registered trademark of Genentech, Inc.
** Open Access
 | ||||
.....................................................
BEV-QNS-AVA (w/o Confirmation): $910.00
BEV-QLS-AVA (w/ Confirmation) : $945.00
Lead time: 2-3 weeks
.....................................................
Drug Measurement
Anti-drug Antibody Measurement
Quantitative
Quantitative
Qualitative
Bevacizumab (Avastin®)
Anti Cancer Drugs
Iwai North America Inc.
541 Taylor Way Suite# 4
San Carlos, CA 94070
Phone : (650) 486-1541
Fax : (650) 394-8638
Open weekdays 9 AM-6 PM (PST)
.........................................................................................................................................................................................................................................................