SHIKARI® Q-TRAS
The Matriks Biotek Trastuzumab ELISA Kit has been developed for the quantitative analysis of free Trastuzumab in serum and plasma samples at high specificity.
SHIKARI® S-ATT
Required Volume
Total Time
Sample
Sample Number
Detection Limit
Spike Recovery
Shelf Life
10ul
70 min
Serum, plasma
96
2ng/mL
85-115%
1 year
The Matriks Biotek anti-Trastuzumab (Herceptin®, Herclon®) Enzyme-Linked-Immuno-Sorbent-Assay (ELISA) Kit is intended for the qualitative determination of antibodies to Trastuzumab (Herceptin®, Herclon®) in serum and plasma.
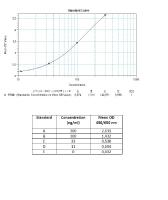
Calibration Curve
SHIKARI® Q-TRAS & S-ATT
Trastuzumab (Herclon®, Herceptin®)
Enzyme immunoassay for the quantitative determination of Trastuzumab (Herceptin®, Herclon®)
Enzyme immunoassay for the determination of antibodies to Trastuzumab (Herceptin®, Herclon®) in serum and plasma
Required Volume
Total Time
Sample
Sample Number
Detection Limit
Spike Recovery
Shelf Life
20ul
140 min
Serum, plasma
96
30ng/ml
85-115%
1 year
TRAS-FD-HH: $910.00 Lead time: 1-2 weeks
TRAS-QLS-HH (w/o Confirmation): $910.00
TRAS-QNS-HH (w/ Confirmation) : $945.00
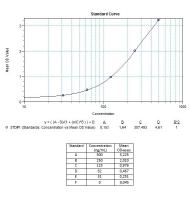
Calibration Curve
TRAS-QLS-HH
TRAS-QNS-HH
Peer Reviewed Journals:
Jonathan GG, Beatriz AÁ, Nazco C GJ, Norberto BL, Fernando GN. Plasma levels of trastuzumab in gastric cancer: Case report. J Oncol Pharm Pract. Sep 23, 2016.**
** Open Access
 | ||||
.....................................................
Lead time: 1-2 weeks
.....................................................
Drug Measurement
Anti-drug Antibody Measurement
Quantitative
Quantitative
Qualitative
Trastuzumab (Herclon®, Herceptin®)
Anti Cancer Drugs
Iwai North America Inc.
541 Taylor Way Suite# 4
San Carlos, CA 94070
Phone : (650) 486-1541
Fax : (650) 394-8638
Open weekdays 9 AM-6 PM (PST)
.........................................................................................................................................................................................................................................................